Medical Device Contract Design & Manufacturing
Resources
Blog

Concept Design to Market Launch - The Biorep Advantage Partner with Biorep on your journey of innovation, where our FDA compliant 5-phase design and development process sets the stage for…

In today's world of fast-paced technological advancements, it's easy to get caught up in the rush of getting products out the door as quickly as possible. While time is certainly…
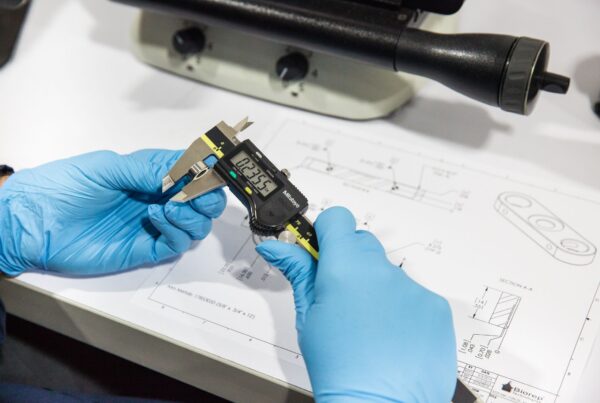
Working with a development firm with a certified quality system saves time and reduces cost. A company’s quality system defines the development, manufacturing, and product lifecycle management procedures that are…